

|
罗瑞
Singlet oxygen-dominated non-radical oxidation process for efficient degradation of bisphenol A under high salinity condition
Rui Luo, Miaoqing Li, Chaohai Wang, Ming Zhang, Muhammad Abdul Nasir Khan, Xiuyun Sun, Jinyou Shen, Weiqing Han, Lianjun Wang, Jiansheng Li*
Abstract
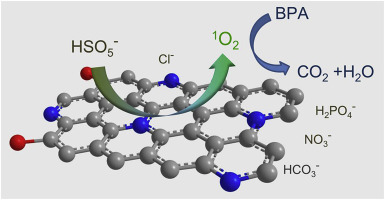
The degradation of organic contaminants under high salinity condition is still a challenge for environmental remediation due to the inhibiting effect resulted from the side reactions between radicals and anions. Here, we demonstrate the non-radical oxidation process via peroxymonosulfate (PMS) activation by metal-free carbon catalyst for efficiently decomposing bisphenol A (BPA) in saline water. The nitrogen-doped graphitic carbon (NGC700) exhibits excellent catalytic activity for depredating BPA at acid and neutral pH. Based on the scavenger experiments and electron paramagnetic resonance (EPR) analyses, the mechanism of catalytic oxidation was elucidated as the non-radical pathway, and singlet oxygen was identified as the primary reactive species. Experiments on the influence of anions (5–500 mM) further show that the inhibiting effect was overcame due to the non-radical process. Interestingly, Cl− markedly facilitated the catalytic performance by generating HOCl in the catalytic process. The results highlight leveraging the non-radical pathway dominated by singlet oxygen to conquer the inhibitory effect of anions in NGC700/PMS system, which represents a crucial step towards environmental remediation under high salinity condition.


 在线咨询
在线咨询



